Part 2 of 3: how does an Iron-Oxide battery cell work, Colorado Women's Alliance Women's Day, and asking others to pay your debts is not fair.
Part 2 of 3: How does the cell in an Iron Oxide Battery work?
This is part 2 of 3. I'm going to lay out what I've been able to find about iron oxide (IOx) batteries--the great rusty hope for renewable energy storage.
You can read this without reading part 1 but there's a lot of technical detail here. If you find yourself getting lost, go back and look over part 1 quickly.
The same applies to part 3.
So how do IOx batteries work? The most common catchy way to put it is that they work by getting electricity from iron rusting. If you're like me, then, the football-field sized electric battery they're going to put in Pueblo comes up in your mind as a giant warehouse with rusty hunks of iron in it and wires running everywhere.
The reality is a lot more technical.
I am not a chemist and this is written for a general audience, so if you are a chemist or highly technical, forgive some physicist's shortcuts. If you want a real challenge and high detail, check out the review article linked first below.
What chemical reaction powers an IOx battery? Take a look at screenshot 1. You may not have been aware, but when iron and oxygen combine, they produce not just rust but 2 electrons are freed up. I want you to note the double-headed arrow too because it's important. It means that this reaction is reversible. If you were to push 2 electrons into rust, you would get back iron and oxygen.
If you connected a wire to this reaction, you could make those electrons do some work for you on their way to ground. Run them through a light to brighten a room. Make them spin a motor that pumps water.
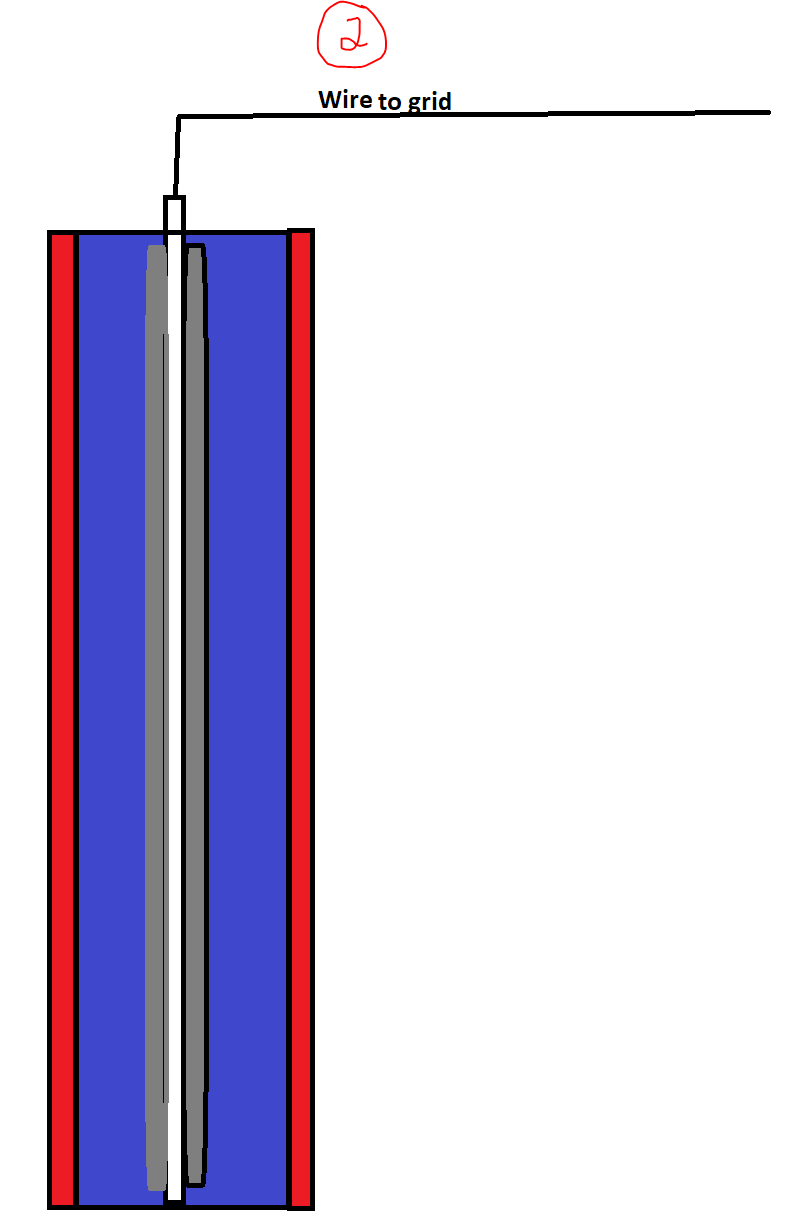
A simplified schematic of a unit cell for an IOx battery is in screenshot 2. If you want a more technical look, check the first link below at the bottom of page 4.
The electrode at the center of the cell is white. This is how the electricity travels out of the battery and up to the wire that connects it to the grid. Coated on the outside of this electrode in grey is the iron. This is immersed in an electrolyte solution (blue) which is held in place by a membrane (red) which is permeable to oxygen and other gases. Outside the red membrane is air. It's open to the air. A battery (as the name suggests) is merely a stack of these unit cells.
How does it work? Take a look at screenshot 3. When the battery discharges, an oxygen molecule which touches the membrane passes through it and gets dissolved in the electrolyte solution. This dissolved oxygen then reacts with the iron to form some kind of iron oxide (there is more than one type). That reaction releases two electrons which travel up the electrode and onto the grid.
Charging the battery would be the reverse. Putting electricity into the battery will break down the rust and leave a dissolved oxygen in the electrolyte which, upon hitting the membrane, will pop out to the atmosphere.
As with everything else human beings undertake, the goal is to maximize the benefit and lower the cost. This leads straight away to a couple positive things about IOx batteries in general: they run on materials that are cheap and plentiful (iron and oxygen) and they do not have the fire risk that lithium ion batteries do.
A good deal of the review paper linked below goes into optimizing these cells and their performance. Again, if you are chemically minded and want a challenge, please give it a read. I can give you a sense of it in rough terms based on my own limited understanding, however.
Let's talk energy and power for a minute (and go back to look at the previous post if you need the background).
One of the big limiting features in any battery is the reaction itself. Chemical batteries run on chemical reactions and these reactions often take a bit of energy to get going. Think of it like a combustion engine: you have to put energy into compressing the air in the cylinder in order to reap the (greater) energy reward when you combust the air with the fuel to drive the piston down.
In the case of an IOx battery, there is some energy cost to trying to get the oxygen through that permeable barrier and into the electrolyte. A good deal of the design challenge in making these cells efficient, then, is to try and figure out a barrier that lets oxygen out of the air and into the solution with as low an energy cost as possible so that the net performance of the battery is bigger.
Another example relates to power. There are a couple drivers of the rate of chemical reactions that are easy enough to understand at a layman's level: temperature and surface area. More on temperature in post 3, but consider surface area. Last time you started a fire you used kindling instead of trying to light a giant log right? Kindling is smaller pieces that have more surface area than a big log so the fire spreads easier through them and the combustion reaction gets off to a quick start.
In an IOx battery, one of the determinants of the power a cell can produce (not energy per se) is the surface area of the iron that is sitting in the electrolyte: more surface, quicker electron production (and also ultimately more energy).
That is another thing you'll see a lot of discussion about in the paper below. What kinds of iron geometries will be best? It seems like there are multiple schemes out there, but in rough terms they seem to converge on the idea of a pebbly surface so there can be lots of iron ready to combine with oxygen.
I.e. expect that as opposed to a sheet of iron.
I hope this post gives a sense of the how the IOx cells work, what they look like, and at least a couple things that determine performance at the basic chemical level.
These are all things that very creative and very smart people are working on right now and I think it's reasonable to assume some headway is being made and has been made. If you look at the date of the review paper below and consider how quickly technology moves these days, you'll see what I mean.
https://www.researchgate.net/publication/266150203_A_Review_of_the_Iron-Air_Secondary_Battery_for_Energy_Storage
On March 10th the Colorado Women's Alliance will celebrate Colorado Women's Day.
If you are a woman interested in getting involved in this state or you know one who is this is a good chance to network, spend time with likeminded people, and learn/be inspired.
I have been on this group's list for a while now. I support what they do and think they do good things. We need more conservative women in this state speaking up and getting involved.
The flyer is attached as a screenshot and the link to register is below.
https://www.zeffy.com/en-US/ticketing/c345f2e3-a3c3-455f-9596-22ddc985852c
It is not fair to ask someone else to pay for your decisions.
There are cases where we as a society have chosen to incentivize certain behavior by forgiving student debt. We should, however, be careful about who we extend this privilege to, and for what reason.
Simply extending this privilege to someone because you like them, because you politically align with them (and possibly want to curry favor) is not fair to those who foot the bill.
I wrote the op ed linked first below to speak out against the bill linked second below.
https://www.coloradopolitics.com/opinion/indebted-adjuncts-should-try-another-career-path-opinion/article_12a4bea8-b187-11ed-88d9-4381a2cd2cf7.html
https://leg.colorado.gov/bills/sb23-084